Protein Interactions at Bio–Nano Interfaces
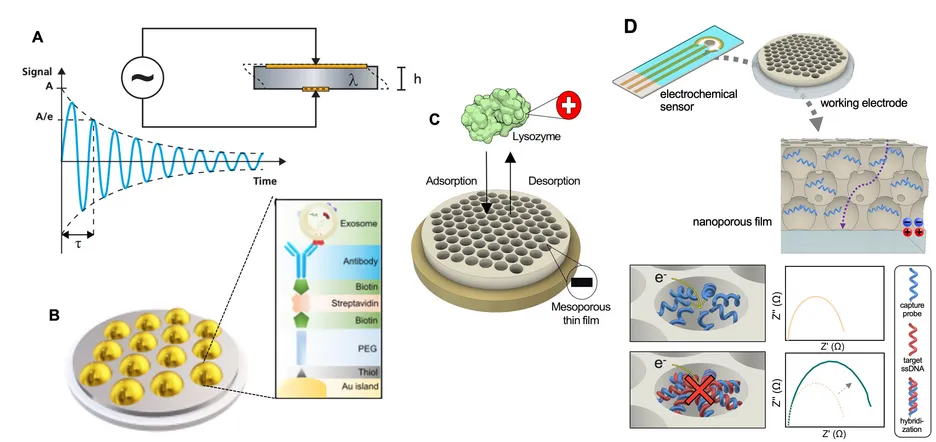
We study protein interactions at bio–nano interfaces with a focus on molecular recognition, surface binding, and interfacial structure–function relationships. A central technique in our work is quartz crystal microbalance with dissipation monitoring (QCM-D), which allows us to monitor adsorption and desorption processes on surfaces in real time. By measuring changes in resonant frequency and energy dissipation, QCM-D provides insights into the mass, viscoelasticity, and structural organisation of adsorbed biomolecules. We use this approach to investigate a range of interfacial phenomena.
We have extended the application of QCM-D to the acoustic immunosensing of extracellular vesicles (EVs) - membrane-bound lipid particles implicated in cancer and neurodegenerative disease diagnostics and of value across various other healthcare and environmental applications. The combined frequency and dissipation signals enables clear discrimination of EVs in complex physiological fluids based on their mass and viscoelastic properties. To further increase analytical power, we integrated QCM-D with electrochemical impedance spectroscopy, creating an electrochemical QCM-D. In recent work, we have used molecular self-assembly to spatially pattern sensor surfaces, improving sensitivity by matching anchor point distribution to analyte dimensions.
A major focus of our group is on mesoporous thin films as engineered interfaces for biointeractions. Using block copolymer templating, we create porous architectures with tunable geometry and surface chemistry. These materials allow us to study processes such as enzyme encapsulation, selective biomolecular adsorption, and controlled release within confined nanoscale environments. By systematically varying pore size, film morphology, and surface charge, we explore how nanoscale confinement influences interfacial behaviour. Building on this, we have recently demonstrated that nanoporous electrochemical biosensors fabricated via bottom-up self-assembly can serve as sensitive platforms for DNA detection. By integrating these materials directly onto electrode surfaces, we achieve highly controlled pore architectures and efficient probe functionalisation, offering a scalable route to rapid and portable diagnostic devices.