Enzymatic de-polymerization of hemicellulose from agricultural residues for xylitol liberation by Aspergillus niger
As a saprophyte specialized on hemicellulose-rich substrates, Aspergillus niger is considered an ideal host for industrial scale D-galacturonic acid (D-GalA) release from pectin-rich biomass such as sugar beet pulp or apple pomace. The Benz lab focusses on strain engineering in A. niger to realize more efficient enzymatic depolymerization of hemicellulose and thereby enabling economically attractive D-GalA, arabinose and xylitol supply from agricultural residues. Amongst targeted transcription factor engineering, selective modifications to the endogenous metabolism of varies carbon sources as well as morphological engineering, also recent technologies for genome editing, such as CRISPR/Cas9, are applied. In close collaboration with the institute for biochemical engineering at the TU Munich, these modifications are thoroughly tested and finally implemented in a robust fermentation and hydrolysis processes for the retrieval of galacturonic acid and pentoses for the following bioconversion.
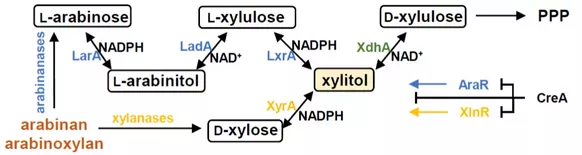
Metabolic engineering of Aspergillus niger for the bio-conversion of arabinose and xylose to xylitol
The degradation of the biomass is carried out by Aspergillus niger and the released pentoses are subsequently converted and metabolized into xylitol within the cells. In order to ensure efficient metabolism of the starting materials, the genetic inventory of the organism is used and expanded by the expression of genes foreign to the species. Particularly important for product-generating catalysis is the adaptation of the redox pool by redirecting the native catabolism of hexoses and pentoses.
Metabolic-engineering of Saccharomyces cerevisae for the bio-conversion of D-galacturonic acid over L-galactono-y-lactone to L-ascorbic acid and ascorbyl palmitate
In the proposed project, L-AA will be produced as a derivative of D-GalA via L-galactono-γ-lactone (GgL), whose production was already established in the first project period. Only one enzymatic reaction is required to convert GgL to L-AA and a proof of concept was achieved in A. niger, albeit with low yields. AP is currently synthesized only via chemical routes and we will attempt to establish its biotechnological production for the first time. Different promiscuous alcohol-acyl transferases will be screened for their ability to catalyze the esterification between the terminal hydroxyl group of L-AA and palmitic acid. Once suitable enzymes are identified, they will be introduced into yeast cells engineered for high level production of fatty acids.
Establishing a bio-fermenter process for industrial scale production of xylitol and ascorbic acid derivates by fermentation of hemicellulose-rich substrates
Analogously to the objectives in the first funding phase, we aim to produce the enzymes with A. niger on-site (step 1) by using a small side stream of SBP as carbon source (and inducer for non-constitutively producing strains). This tailor-made enzyme cocktail will then be separated from the fungal biomass and used for the deconstruction of the major SBP biomass in a cell-free hydrolysis step (step 2). The resulting slurry will be solid-liquid extracted and the sugar-rich hydrolysate used as feed for the bioconversion strains (step 3). Over the course of the project, we will work towards a consolidation of this 3-step process into a 2-step or even one-pot process.