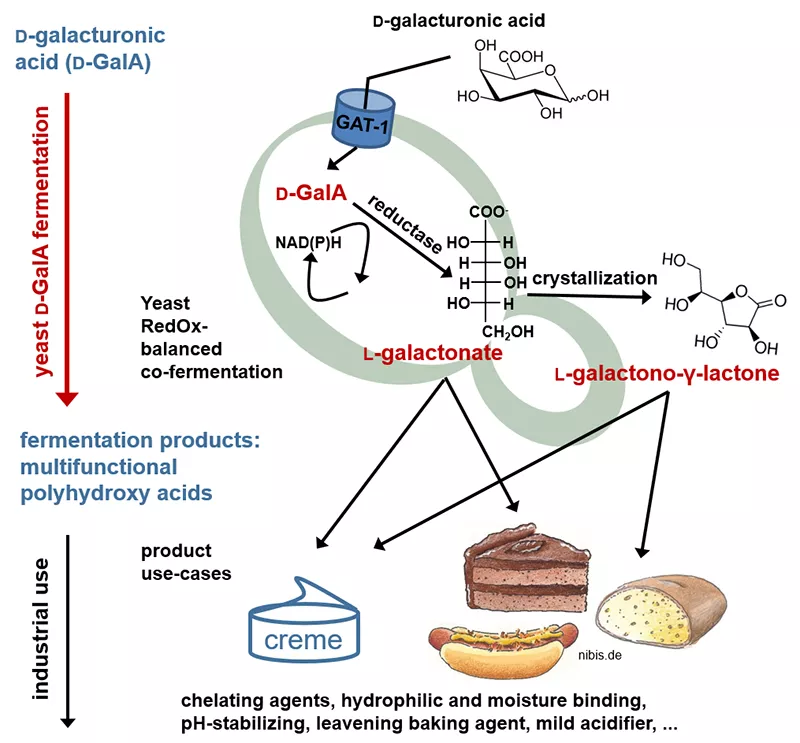
D-galacturonic acid supply from agricultural waste
Enzymatic de-polymerization of pectin-rich, residual agricultural material streams for D-galacturonic acid retrieval using Aspergillus niger
As a saprophyte specialized on pectin-rich substrates, Aspergillus niger is considered an ideal host for industrial scale D-galacturonic acid (D-GalA) release from pectin-rich biomass such as sugar beet pulp or apple pomace. The Benz lab focusses on strain engineering in A. niger to realize more efficient enzymatic pectin depolymerization and thereby enabling economically attractive D-GalA supply from agricultural residues. Amongst targeted transcription factor engineering, selective modifications to the endogenous metabolism of galacturonic acid as well as morphological engineering, also recent technologies for genome editing, such as CRISPR/Cas9, are applied. In close collaboration with the institute for biochemical engineering at the TU Munich, these modifications are thoroughly tested and finally implemented in a robust fermentation and hydrolysis processe for the retrieval of galacturonic acid and the following bioconversion.
Establishing a bio-fermenter process for industrial scale D-galacturonic acid supply from pectin-rich substrates using Aspergillus niger
The objective of this research project is the reaction engineering analysis of recombinant A. niger strains provided by the Benz lab. The studies are carried out in stirred tank bioreactors under defined conditions. Parallel controlled bioreactors are used for the comparison of different recombinant strains as well as respective fermentation and hydrolysis conditions. Based on the results of these studies, scalable and efficient processes are designed and validated on a liter-scale for the production of pectinases as well as D-galacturonic acid from pectin rich residues. Of particular interests are the studies on simultaneous pectinase production and hydrolysis as well as a potential process integration with the subsequent bioconversion of the produced D-GalA.
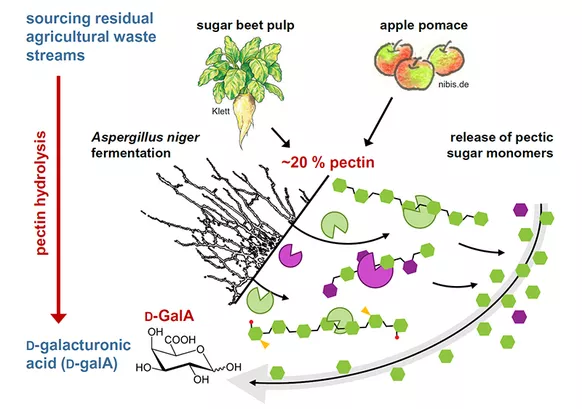
D-galacturonic acid conversion to polyhydroxy acids
Metabolic engineering of Saccharomyces cerevisae for bioconversion of D-galacturonic acid to polyhydroxy acids
We aim to engineer this yeast for biotransformation of D-GalA to L-galactonate (L-GalOA), which has potential applications as chelator, moisturizer, pH-stabilizer and leaving agent in food and cosmetic industry. This requires the expression of heterologous D-GalA transporters and reductases, but also extensive interventions into the central carbon metabolism of the host cell. Previous attempts to construct D-GalA utilizing S. cerevisiae strains have revealed that the higher oxidation state of D-GalA (compared to sugars) is one of the challenges for its funneling into the endogenous metabolism of yeast (Biz et al., 2016), since surplus reduction equivalents are required. These can be derived from the fermentation of sugars present in the pectin biomass, mainly glucose, galactose and arabinose by blocking the ethanol formation. In this way, a nearly complete valorization of the pectin feedstock, which is currently underused, can be achieved.
Establishing a bio-fermenter process for industrial scale D-galacturonic acid conversion to polyhydroxy acids using Saccharomyces cerevisae
The objective of this research project is the reaction engineering analysis of the provided novel, recombinant Saccharomyces cerevisiae strains under controlled reaction conditions in stirred-tank reactors. Miniaturized parallel bioreactors will be used for comparison of different recombinant strains. Based on the derived data from the reaction engineering analyses, scalable and efficient processes for the whole-cell biotransformation of D-galacturonic acid will be established and verified on the litre-scale. Subsequently, methods for the isolation, purification and lactonization of L-galactonate are developed. Of particular interest are the studies on a potential process integration of the biotransformation and the former hydrolysis of pectin rich residues.